Risk Management Plan
Reading time: 2 min
NERLYNX® Risk Management Plan
Pierre Fabre is committed to make sure that patients can get the full benefit of their treatment. A Risk Management Plan (RMP) agreed with EMA has been set up in Europe.
Specific educational materials are intended to all treating physicians who are expected to prescribe NERLYNX® and to all patients who are expected to get treated with NERLYNX®. They are developed in local language and approved by each Health Competent Authority, as required in the RMP. Thus, educational materials were designed to increase awareness of diarrhoea-induced incidence and help with its management, to be offered to treating physicians and patients prior to treatment initiation.
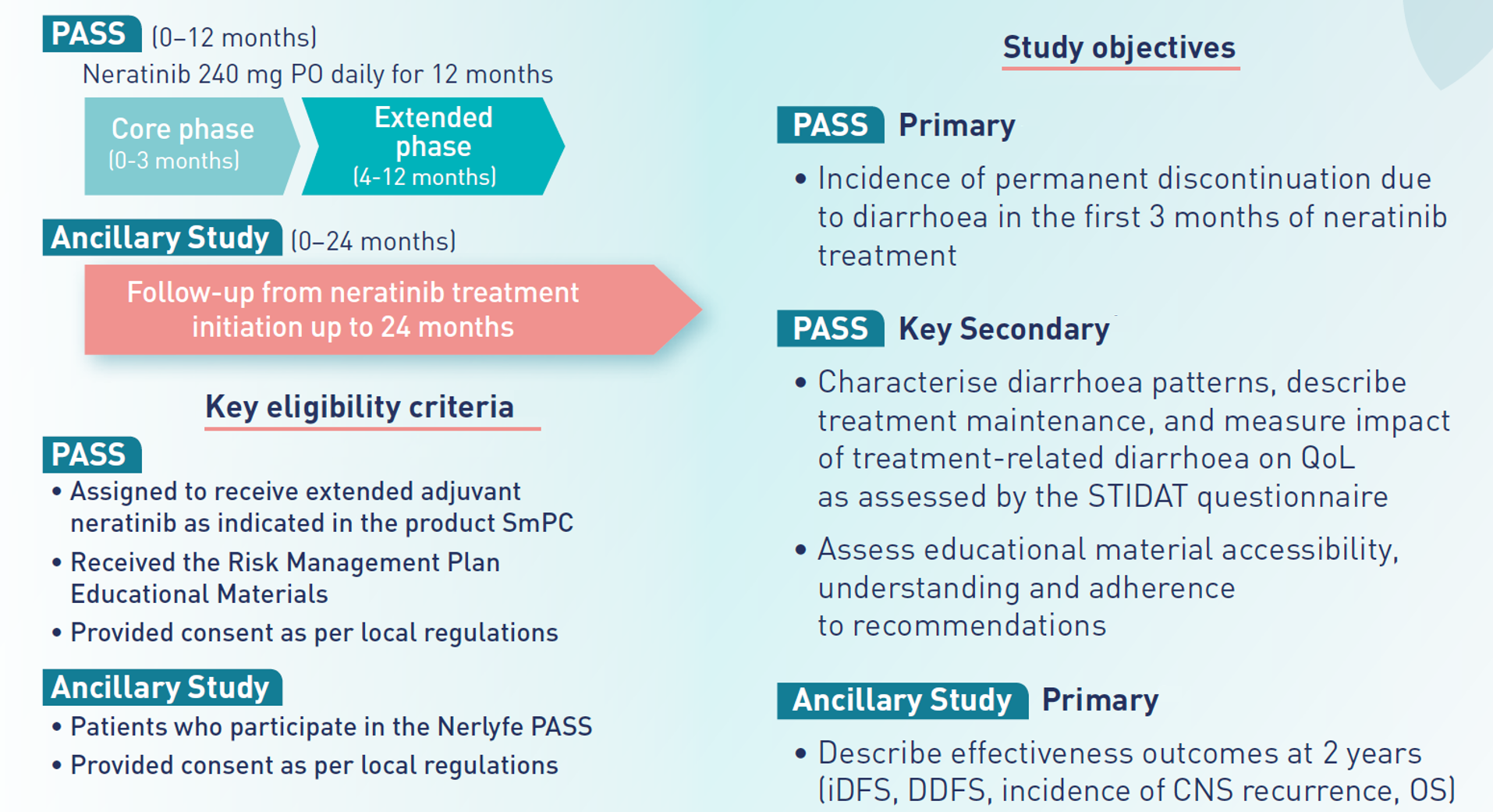
In addition, the RMP includes the conduction of post-authorization safety studies (PASS) to observe the incidence of permanent discontinuation due to diarrhoea in the routine clinical practice and to evaluate if the upfront education of healthcare professionals and patients can impact on diarrhoea prevention and management. NERLYFE is a European, prospective, observational, PASS to investigate real-world incidence and management of diarrhoea under NERLYNX® as extended adjuvant treatment.
Study is active
Prospective observational safety study in adult breast cancer patients treated with neratinib
in extended adjuvant in a real-world setting38
To describe
the incidence of discontinuation due to diarrhoea within the first 3 months of treatment with neratinib.38
To assess
the impact of the information provided in the educational materials according to Risk Management Plan engagement.
EU PAS Register number: EUPAS41584.38
For more information about Nerlyfe, please send an email to: nerlyfestudy@pierre-fabre.com