Dosing
Reading time: 4 min
NERLYNX® is to be taken orally, once a day, as six 40 mg tablets, continuously for one year5
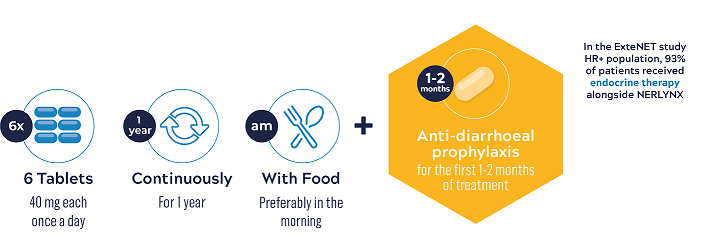

NERLYNX® should be taken with food, preferably in the morning every day, continuously for 1 year

If a dose of NERLYNX® is missed or vomited, inform patients that the missed dose should not be replaced and to resume NERLYNX® with the next scheduled daily dose

Tablets should not be chewed, crushed, dissolved or split prior to swallowing

Grapefruit or pomegranate may inhibit CYP3A4 and/or P-gp and should be avoided during treatment with NERLYNX®

NERLYNX® does not require any special temperature storage conditions
Adjusting daily dose can improve tolerability and adherence5
Management of some adverse reactions may require dose interruption and/or dose reduction.5
In case of Grade 3 toxicity:
Stop NERLYNX® until recovery to Grade ≤ 1 or baseline within 3 weeks. Then resume NERLYNX® at the next lower dose level. If Grade 3 toxicity does not recover within 3 weeks, discontinue permanently. NERLYNX® should be discontinued for patients that are unable to tolerate 120 mg daily or who fail to recover to Grade 0–1 from treatment toxicity, or for toxicities resulting in a treatment delay >3 weeks.5
Dose adjustments for specific populations5
-
Elderly
No dose adjustment is required.
There is no data in patients ≥85 years of age.
-
Renal impairment
No dose adjustment is required for patients with mild to moderate renal impairment.
NERLYNX® has not been studied in patients with severe renal impairment (eGFR ≤29 mL/ min/1.73 m2) including patients on dialysis. Treatment is not recommended for these patients.
-
Hepatic impairment
No dose adjustment is required for patients with Child-Pugh A or B (mild to moderate).
Dose adjustments for general hepatotoxicity5
Hepatotoxicity has been reported in patients treated with NERLYNX®.
Liver function tests including alanine aminotransferase (ALT), aspartate aminotransferase (AST), and total bilirubin should be monitored at 1 week, then monthly for the first 3 months and every 6 weeks thereafter while on treatment or as clinically indicated.5
See dose adjustment for hepatotoxicity
hepatotoxicity & action