HER2+ / HR+ early breast cancer
Breast cancer epidemiology
Breast cancer is the most common cancer in women, with more than 2 million new cases each year worldwide.
Amplification or overexpression of HER2 occurs in approximately 15–30% of breast cancers.15
Around 80% of breast cancers test positive for ER, with 65% of these also testing positive for PR.29
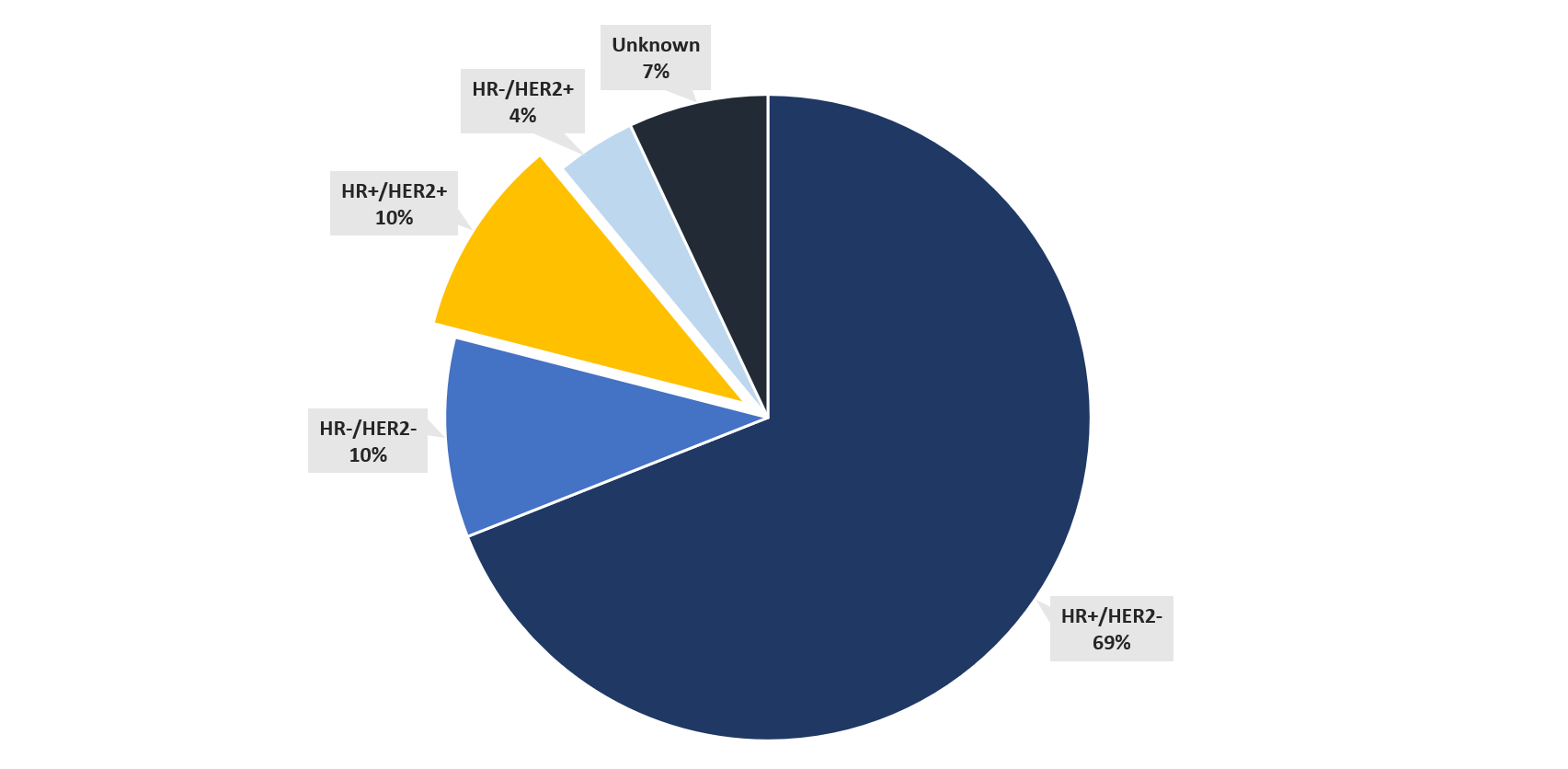
Around 10% of breast cancers are HER2+/HR+.30
Breast cancer in females by subtype (%)30 :
After completion of trastuzumab-based therapy, your HER2+/HR+ early breast cancer patients are still at risk of recurrence1-3,6,7,31-36
Without further treatment, 20-30% of patients with HER2+/HR+ disease will experience disease recurrence within 10 years of completing trastuzumab-based therapy. 1-3,6,7,31-36
| Residual risk of recurrence for HER2+/HR+ (%) | 3 years | 5 years | 6 years | 8 years | 10 years |
|---|---|---|---|---|---|
| EBCTCG meta-analysis31,a (adjuvant trastuzumab) |
- | 14 | - | - | 20 |
|
HERA6,33,b |
10 | - | - | - | 28 |
|
APHINITY32,34,35,b |
5 | - | 9 |
11 |
- |
| KATHERINE7,c (post-neoadjuvant T-DM1) |
9 | - | - |
- |
- |
| ExteNET control arm in HER2+/HR+ less than one year since trastuzumab therapy population1,d (adjuvant trastuzumab) |
- | 14 | - | - | - |